The Science of Moisture-Wicking
你好!
I write to you from China, where we are working on the production of the heating system and microcomputer for the Mercury Jacket.
It’s currently 89ºF and 78% Relative Humidity. Suffice to say, it’s hot and sweaty!
We focus on creating garments using moisture-wicking fabrics to help stay cool in exactly this kind of environment. Moisture-wicking fibers not only keep us dry and help prevent sweat stains, but they also promote evaporative cooling - a process that naturally keeps our body-temperature within a comfortable range. Moving moisture away from our skin is critical to cooling off. Also, feeling clammy is simply an uncomfortable sensation.
TL;DR: We sweat to cool off through evaporative cooling. There are many factors that contribute to accelerating this “dry” environment.
Why do we sweat?
When our bodies are hot, they sweat in order to cool off through a process called evaporative cooling. In just the same way that PCMs in the Apollo absorb heat when the paraffin wax goes from solid to liquid form (a phase change), water also absorbs heat when it evaporates from liquid water to moisture vapor (steam). Sweating is an especially good way to cool off because body heat is absorbed into the steam, allowing it to flow away from our body into the air.
To illustrate this point, think about heat vs. humidity. A 90º day in Atlanta (80% humidity) feels much hotter than a 90º day in Phoenix (7% humidity) because of the humidity. Relative humidity is a measure of how saturated the air is with water with respect to the maximum amount water it can hold at a certain temperature, so if the air is already full of water, then it will be slower at absorbing evaporated sweat. In humid environments, Our bodies heat up more, we sweat more, and there’s nowhere for the heat to go. It’s a vicious cycle!
In other words, sweating and moving moisture away from our skin is critical to cooling off.
How do different materials react with water?
The Shape of Water
The physical shape of water molecules is distinctive, allowing us to develop fabrics that react to moisture contact in predictable ways.
Water is polar and likes polar materials: Water is an interesting molecule, it looks like Mickey Mouse: the ears are hydrogen and the face is oxygen, which has a slightly negative charge. The result is polarity - meaning that water molecules stick like magnets to other polar molecules.
Hydrophilic
“Water-loving” fibers, have the ability to absorb liquid water into their fiber - much like cotton. This characteristic largely stems from the fact that cotton is comprised of cellulose, which is also very polar. It has lots of charged points for water to adhere to, which explains why cotton can absorb 25% of its weight in water!
Hydrophobic
“Water-hating” fibers repel water. Polyester, one of the most hydrophobic fibers, is derived from oil, which is very non-polar, so water molecules have a hard time adhering to the molecule. (Remember how oil and water don’t mix!)
Where does the Water Go?
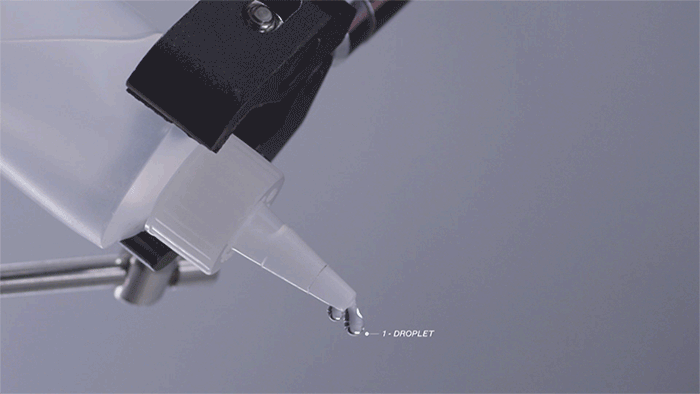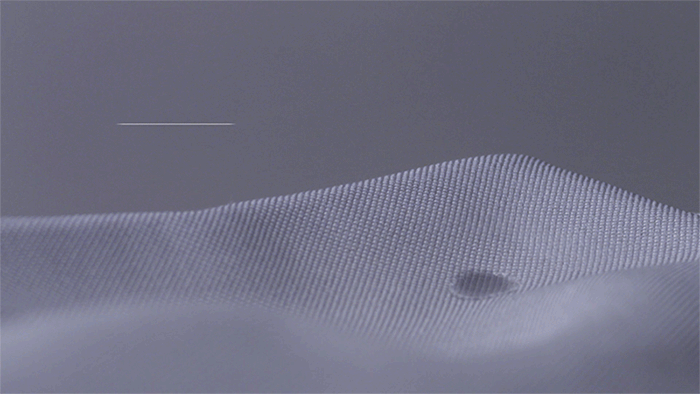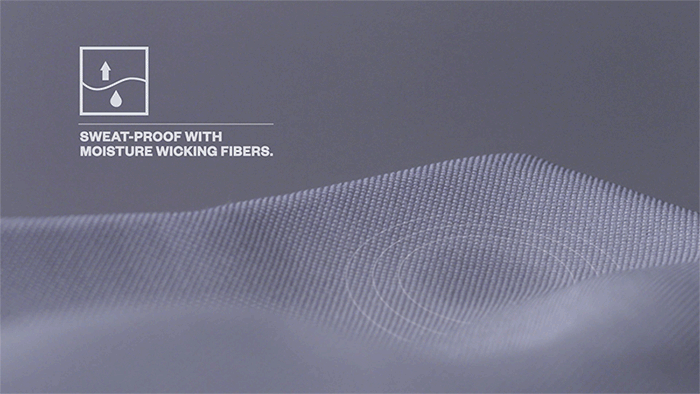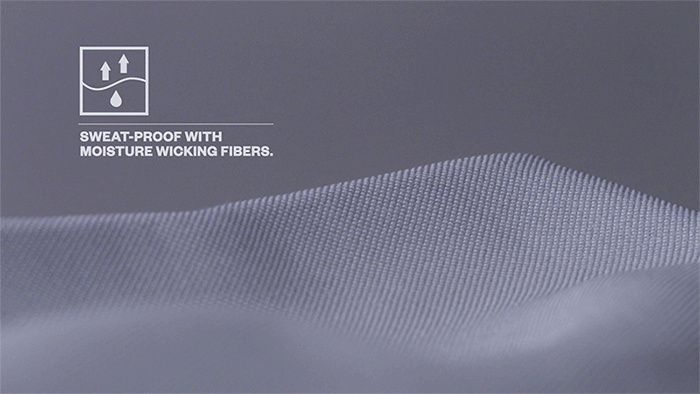
Liquid Moisture
Moving water through a fabric is critical to efficient evaporation, and there a several ways we move water away from the body and spread it out to evaporate faster.
Vertical Transfer
First, we must pull water away from the body to the surface where it can evaporate faster.
Capillary Action: Capillary Action is when water is drawn upwards. The Apollo fabric uses a pique knit, which has small holes next to skin and larger holes on the surface. This structure pulls moisture to the surface.
Push/Pull: Hydrophilic/hydrophobic plaiting creates a push-and-pull effect. By putting a hydrophobic material like nylon or polyester next to skin and a hydrophilic material like cotton on the surface, we create an effect that is biased towards pulling moisture towards the surface, where it can evaporate. We use this technique in the Atlas Tees.
Horizontal Transfer
After pulling moisture away from the skin we must spread it over a larger surface area to dry faster. Yarn Shape affects the way moisture spreads. For example, small structures on the surface of polyester pull moisture down the length of the yarn. Cotton, on the other hand, absorbs all of the moisture in one spot, reducing the surface area - so it drys slower.
Moisture Vapor Regain & Hygroscopic Fibers
Hydrophobic materials like polyester, would seem ideal on their own. However, they are optimized for evaporating liquid sweat, especially with significant air flow. That’s why synthetics have thrived in running apparel and other athletic gear. But what about when you’re sweating on a plane for several hours?
Hygroscopic Fibers: “Moisture regain” is the ability to first absorb, then release that moisture vapor into the air. Polyester has 0% moisture regain because it doesn’t absorb water at all. Cotton has 8.5% moisture regain, meaning that once wet, it only releases a small amount of moisture.
The optimal fibers typically blend these material properties and have higher moisture regain. Wool, for example, has 17% moisture regain - twice that of cotton!
Breathability: When people refer to “breathability” what they often sense is a combination of the ability for air to flow through a fabric and also it’s moisture regain.
So what’s the best way to manage moisture?
Material Comparison
We often like to label fibers in their extremes: hydrophobic vs. hydrophilic. In reality, each fiber is different and lays in different parts of the spectrum - and it’s the ones in the middle that have a combination of features that work best!
As we think through long-lasting comfort in future fiber blends, finding this ideal combination of hydrophobic properties for evaporation and high moisture regain for vapor steam will be critical.